Avoiding hemodynamic instability
Over 75" is the fastest growing age group among dialysis patients. These patients suffer from comorbidities that not only affect survival, but also the tolerance of extracorporeal renal replacement therapy.
These include diabetes, heart failure, valve diseases, arteriosclerosis and malnutrition. Within even the first year of dialysis, many patients lose their independence and require nursing assistance. The rapid deterioration of these patients' general condition is due not only to the spontaneous progression of the underlying illnesses, but also to factors inherent to dialysis, which are summarized under the umbrella term "tolerance of hemodialysis therapy". Of particular importance in this context is hemodynamic stability during dialysis, which refers to maintaining normal blood pressure and avoiding hypotension.
The consequences of hemodynamic instability with intradialytic hypotensive phases are far-reaching, with patients complaining of exhaustion and fatigue following dialysis, spending the rest of the day in bed and skipping meals. The consequences are far-reaching, prolonged immobility following dialysis in conjunction with inadequate food intake often leads to a loss in muscle mass, which increases the risk of falling, and the need for assistance and even depression develop more quickly. In the end, the complete clinical picture of frailty develops, resulting in decreased social contact. This makes the overall situation for the entire family increasingly stressful or no longer manageable. The progressing physical decline and increasing need for assistance results in a move into a nursing facility. The complex interconnectedness of the various aspects makes it clear how important it is to avoid hemodynamic instability during dialysis
Clinically, hemodynamic instability is characterized by symptomatic and, even more significantly, asymptomatic hypotension; these are the most common complications of dialysis treatment. Symptomatic hypotension is characterized by a drop in systolic blood pressure to below 100 mmHg, accompanied by complaints such as restlessness, nausea, loss of consciousness, cramping or severe arrhythmia. Patients prone to symptomatic hypotension have a significantly greater mortality risk. Symptomatic hypotension can, in principle, be largely avoided by regularly checking and adjusting the target weight and with a customized, low ultrafiltration rate.
Considerably more difficult to avoid is asymptomatic hypotension, in which systolic blood pressure drops by at least 20 mmHg but not below 100 mmHg and is accompanied by a relevant malperfusion of the intestines, heart and brain without the patient being symptomatic. The more long-term consequences of recurring tissue hypoxia are: accelerated decline in residual renal clearance, silent myocardial ischemia, chronic inflammation due to increased intestinal wall permeability to bacterial toxins and development of depression up to dementia symptoms. Elderly dialysis patients appear to be affected more than younger patients in terms of the frequency and extent of hemodynamic instability. Two factors make elderly dialysis patients especially prone: first, organ systems that have been previously damaged by comorbidities; second, a limited ability of the body to maintain essential organ perfusion in the event of hypotension.
Triggers of hypotension
Typical triggers of hemodynamic instability are fluctuations in intravascular blood volume. Systolic blood pressure is affected by a number of factors, of which intravascular blood volume along with cardiac output and peripheral resistance are essential factors. During dialysis, ultrafiltration removes water from the blood. This would rapidly reduce blood volume if the water was not quickly pushed from the extravascular space into the intravascular space in sufficient quantity. This process is called refilling. An imbalance between ultrafiltration rate (UFR) and refilling rate causes a constriction of blood volume.
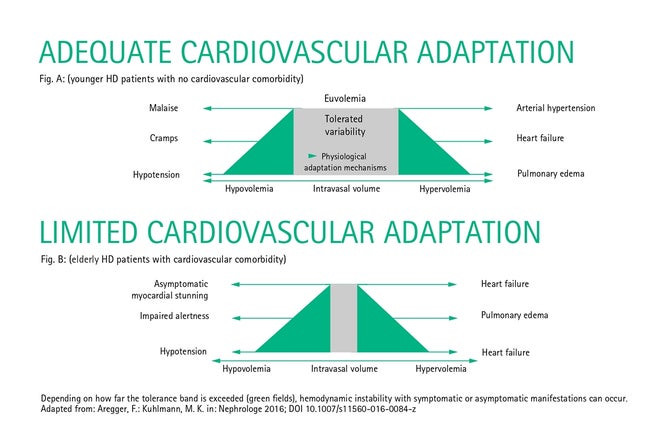
In younger dialysis patients with no relevant cardiovascular comorbidities, this is offset by physiological adaptation reactions that maintain an adequate blood and oxygen supply to the organs. These include an increase in heart rate, an increase in cardiac output and the vasoconstriction of smaller arteries and arterioles, as well as venous conduits. In addition, nearly all organs have the ability to autoregulate their perfusion in the event of blood pressure fluctuations, maintaining perfusion pressure in the organ by reducing vascular resistance in the organ. Only when these compensatory and autoregulatory mechanisms are no longer able to adequately react to intradialytic fluctuations in blood volume do organ perfusion disorders or clinical symptoms such as malaise, cramps and hypotensive episodes occur (Fig. A).
Causes are varied
The reduced tolerance range of intravascular volume fluctuations has many causes. Elderly dialysis patients already suffering from diabetes often experience autonomic nervous disorders that are expressed as limited heart rate variability and insufficient increase in cardiac output in response to an intradialytic contraction of blood volume. Reduced vasoconstriction can also have a neurogenic cause or be due to increased vascular stiffness. Even the refilling process can be impaired. The moving of water and electrolytes from the extravascular space to the intravascular space is primarily controlled by changes in oncotic pressure and plasma osmolarity. Oncotic pressure in blood plasma is determined by the concentration of proteins, specifically albumin, in the blood serum, with a slight increase having a vortex effect on the fluid in the extravascular space. If the albumin level is low, as is often the case in elderly patients in an insufficient nutritional condition, the oncotic pressure is greatly reduced and refilling is limited.
The second factor, plasma osmolarity, is primarily determined by the blood concentrations of sodium, glucose and urea—substances whose concentrations change during dialysis treatment. A rapid reduction in plasma osmolarity during dialysis also impairs the refilling process. In addition, cardiovascular comorbidities such as CAD, heart failure, valve diseases or stenoses in major arteries help make it more difficult to adapt to volume fluctuations. Another mechanism is the increase in body temperature during dialysis, in consequence of which peripheral skin vessels dilate and result in a relative drop in blood volume.
From the relationships described above, it becomes clear that hemodynamic instability occurs in elderly, multimorbid patients with limited reserve from a minor drop in intravascular volume and that these patients accordingly develop asymptomatic and symptomatic hypotension sooner (Fig. B).
Steps to improve hemodynamic instability
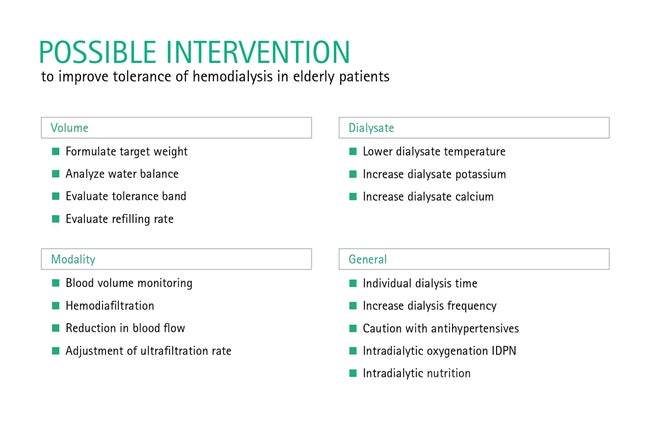
The goal of prescribing dialysis for elderly patients is a hemodynamically neutral treatment without relevant perfusion disorders in the various organ systems. Primarily, the target dialysis weight needs to be accurately determined. In addition to clinical parameters such as a physical examination, the diameter of the vena cava and a chest X-ray, modern bioimpedance technology should be used. Furthermore, an impression of the patient's tolerance range of fluctuations in blood volume should be obtained. This is done by monitoring changes in blood volume online during ultrafiltration. A sudden intradialytic drop in blood volume suggests an imbalance between UFR, refilling rate and physiological adaptation mechanisms, and is cause to reduce the UFR. Modern dialysis machines offer integrated systems that almost continuously monitor blood volume and automatically respond to changes with stored algorithms.
In general, it is recommended that the UFR for elderly patients not exceed 13 mL/kg/h and not exceed 10 mL/kg/h in case of hemodynamic instability. If tolerance to volume fluctuations is low, it may be necessary to further limit fluid intake or increase the frequency of dialysis. Reducing the dialysate temperature by 0.5 °C below the patient's current body temperature can prevent the body temperature from rising; this significantly reduces both intradialytic hypotension as well as silent myocardial and cerebral ischemia. In order not to lower plasma osmolarity too much, efforts should be made to preserve a neutral sodium balance and individually adjust the dialysate sodium concentration (DNa) to the predialytic serum sodium concentration. In the event of hemodynamic instability, increasing the DNa can be helpful. Also increasing the dialysate calcium concentration (DCa) can have a positive effect on hemodynamic instability, therefore the DCa should not be below 1.25 mmol/L.
A sudden drop in plasma osmolarity can be triggered by intensive dialysis in patients with reduced muscle mass or by the elimination of glucose in patients with poorly managed diabetes. In these cases, it may be worthwhile to reduce the blood flow rate or use low-flux instead of high-flux dialyzers. Hemodiafiltration is considered more hemodynamically tolerable and is an alternative. Blood pressure-reducing medication should not be taken before or after dialysis on dialysis days; a predialysis blood pressure around 150 mmHg is considered desirable. The principles of "gentle" dialysis are summarized in Table 1 and generally also apply to younger dialysis patients in order to avoid subclinical tissue hypoxia in the kidneys, intestine, liver, heart and brain. If all of these steps are unable to achieve hemodynamic stability, the use of peritoneal dialysis may ultimately be considered, which is often well-tolerated by hemodynamically unstable patients.